

They are just different ways of keeping track of the electrons transferred during the reaction. There's no real difference between the oxidation number method and the half-reaction method. Now try to balance the equations in the link below (answers included).

Place these numbers as coefficients in front of the formulas containing those atoms.ĢHNO₃ + 3H₃AsO₃(aq) → 2NO(g) + 3H₃AsO₄(aq) + H₂O(l)īalance all remaining atoms other than H and O. This gives us total changes of -6 and +6.
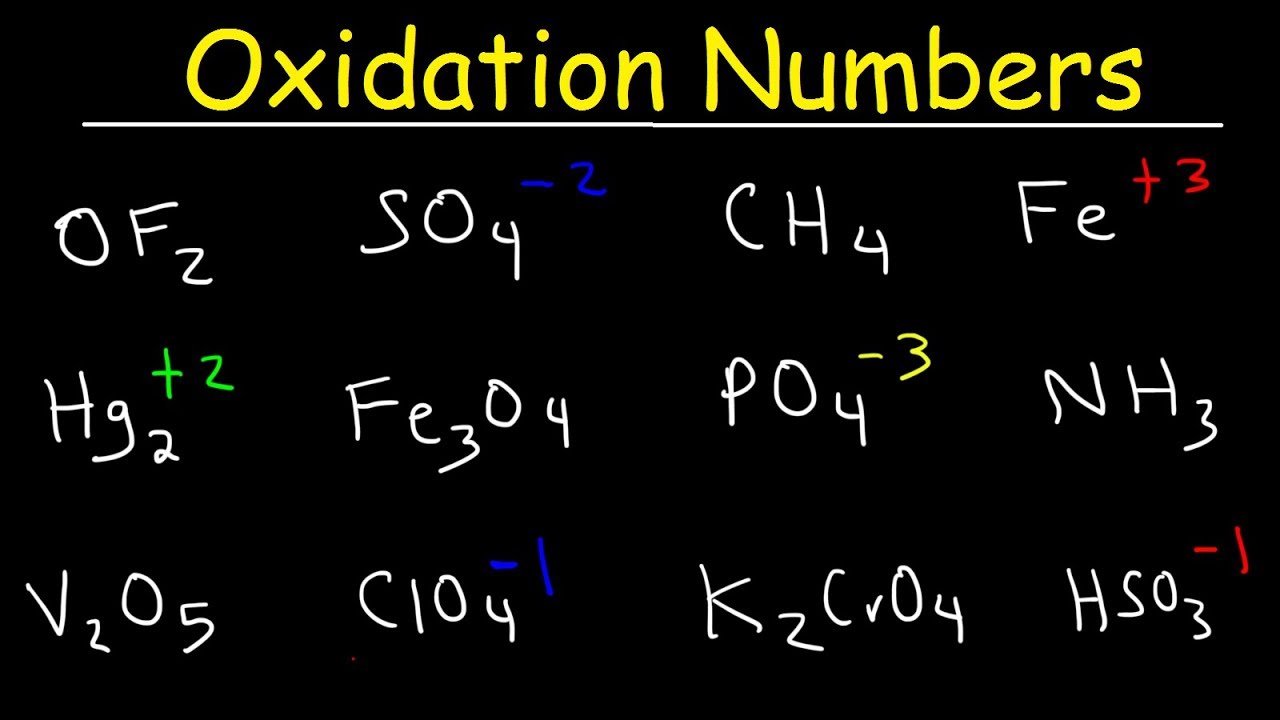
We need 2 atoms of N for every 3 atoms of As. Make the total increase in oxidation number equal to the total decrease in oxidation number. Right hand side: N = +2 O = -2 H = +1 As = +5ĭetermine the change in oxidation number for each atom that changes. Left hand side: H= +1 N= +5 O = -2 As = +3 Identify the oxidation number of every atom.


 0 kommentar(er)
0 kommentar(er)
